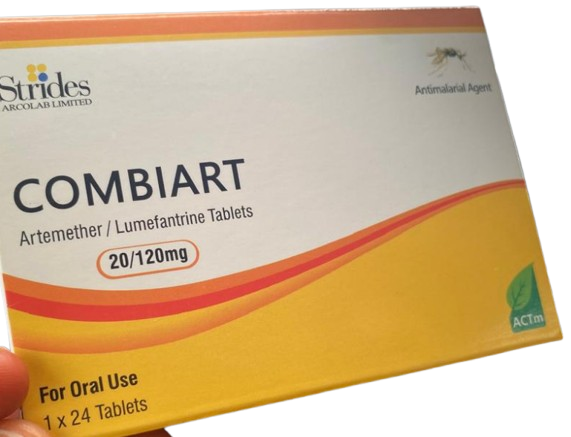
The National Agency for Food and Drug Administration and Control (NAFDAC) has alerted the public to the circulation of counterfeit Combiart Dispersible Tablet 20/120mg in Nigeria. The product, manufactured by Strides Arcolab Limited in India, was discovered in the Federal Capital Territory (FCT) and Rivers State during surveillance activities.
Laboratory analysis revealed that the counterfeit product contains zero active pharmaceutical ingredients (APIs) and has two different date markings. Furthermore, NAFDAC’s database confirmed that the product’s license has expired, and the registration number on the product is incorrect.
The Combiart Dispersible Tablet 20/120mg is an antimalarial medication used to treat malaria, a red blood cell infection transmitted by mosquitoes. However, this medicine is not intended for severe or complicated malaria cases.
NAFDAC warns that counterfeit or falsified medicines pose significant health risks, as they do not meet regulatory standards. The use of such products can lead to serious health consequences, including death.
Product Details:
- Brand Name: Combiart Dispersible Tablet 20/120mg
- Generic Name: Artemether + Lumefantrine 20/120mg Dispersible Tablet
- Batch No: 7225119
- NAFDAC Reg No: A11-0299
- Manufacturing Date: June 2023 and Feb 2023
- Expiry Date: May 2026 and June 2026
- Manufacturer’s Name and Address: Strides Arcolab Limited, 36/7, Suragajakkanahalli, Indlavadi Cross, Anekal Taluk, Bangalore- 562 106, India
NAFDAC advises the public to exercise caution and vigilance when purchasing medicines, ensuring they only buy from authorized dealers. If you suspect a counterfeit product, report it to NAFDAC immediately




Add Comment